The National Agency for Food and Drug Administration and Control (NAFDAC) has officially released the names of medical and pharmaceutical products that have either been withdrawn, suspended, or cancelled from circulation in Nigeria.
In a statement published on its official website on Tuesday, NAFDAC confirmed that these products are no longer approved for manufacture, importation, exportation, distribution, sale, advertising, or use within the country.
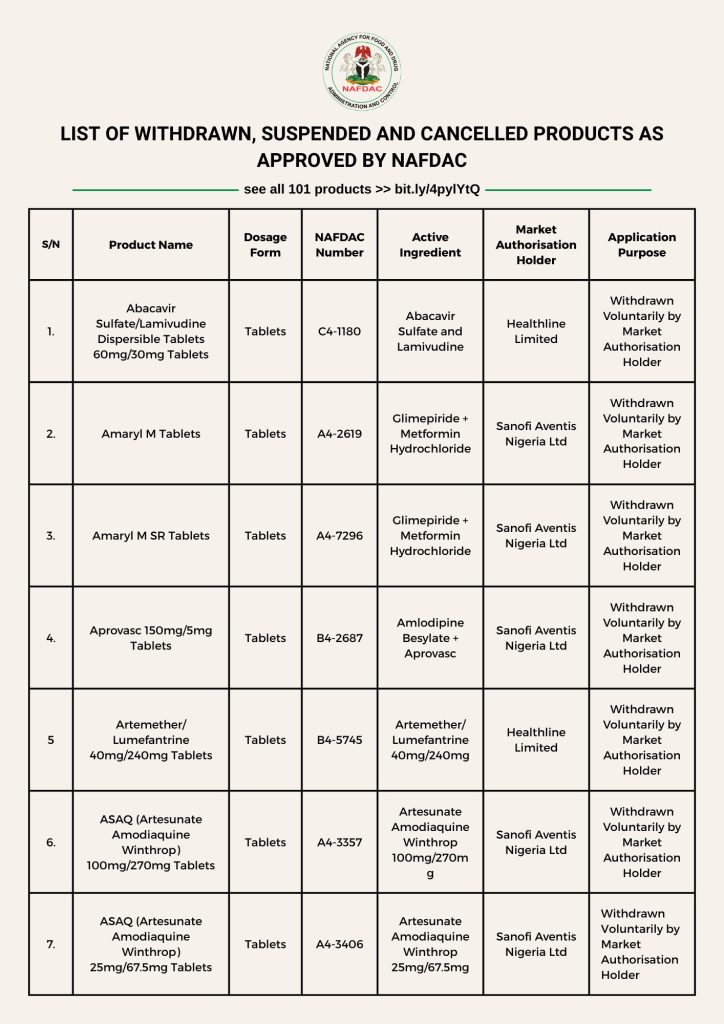
Highlighted Products Affected
Among the affected items are:
Artemether/Lumefantrine 40mg/240mg Tablets
Amaryl M SR Tablets
Abacavir Sulfate/Lamivudine Dispersible Tablets (60mg/30mg)
Aprovasc 150mg/5mg Tablets
ASAQ (Artesunate Amodiaquine Winthrop) 100mg/270mg Tablets
Betopic Eye Drops
Efavirenz 600mg Tablets
Flagyl Suspension
Iliadin Adult 0.05% Metered Nose Spray
Invanz 1g Injections
Invega (Paliperidone) 3mg Extended Release Tablets
Why Products Lose Approval
According to NAFDAC, product registration may be:
Withdrawn – when the market authorization holder voluntarily discontinues the registration.
Suspended – when conditions tied to the original registration license are no longer fulfilled.
Cancelled – when NAFDAC formally revokes the certificate, prohibiting the product from circulation.
Wider Public Health Context
This announcement comes less than 24 hours after NAFDAC unveiled a comprehensive national strategy to eliminate industrially produced trans-fatty acids (TFAs) from Nigeria’s food supply chain, signaling the agency’s stronger commitment to protecting public health.
The full list of the 101 withdrawn, suspended, and cancelled products can be accessed on NAFDAC’s official website.


